Anaerobic breakdown of molecules
by Admin
Posted on 13-10-2023 05:12 PM
.PNG)
What is the function of fermentation? fermentation enables cells to produce chemical energy from the breakdown of sugar, e. G. Glucose, without the help of oxygen. That gives anaerobic (obligate, facultative, or aerotolerant) organisms the advantage of thriving in anoxic (without oxygen) environments that would rather be harsh for aerobic organisms. Examples of anoxic environments are mud, soil, and hydrothermal vents deep under the sea. The anaerobic bacteria that can thrive in these environments are
essential
for their ecological niche.
 They ferment molecules to derive energy and, in return, they produce byproducts released into the environment. Their byproducts may be used by other organisms or may be returned to the environment as a form of nutrient cycling.
They ferment molecules to derive energy and, in return, they produce byproducts released into the environment. Their byproducts may be used by other organisms or may be returned to the environment as a form of nutrient cycling.
Fermentation is an anaerobic breakdown of carbohydrates in which an organic molecules the final electron acceptor and does not involve an electron transport system. Fermentation is a partial breakdown of glucose producing only 2 net atp's per glucose by way of substrate-level phosphorylation, involves only glycolysis, and is found in anaerobic and facultative anaerobic bacteria. The overall reaction is glucose (6c) + 2 nad+ +2 adp +2 inorganic phosphates (pi) yields 2 pyruvate (3c) + 2 nadh + 2 h+ + 2 net atp. Glycolysis also produces a number of key precursor metabolites. Some fermentation end products produced by microorganisms are very beneficial to humans and are the basis of a number of industries (brewing industry, dairy industry, etc.
F ood N etwork S olution
Fermentation is a natural process.
people
applied fermentation to make products such as wine, mead, cheese, and beer long before the biochemical process was understood. In the 1850s and 1860s, louis pasteur became the first zymurgist or scientist to study fermentation when he demonstrated fermentation was caused by living cells. However, pasteur was unsuccessful in his attempts to extract the enzyme responsible for fermentation from yeast cells. In 1897, german chemist eduard buechner ground yeast, extracted fluid from them, and found the liquid could ferment a sugar solution.
.PNG)
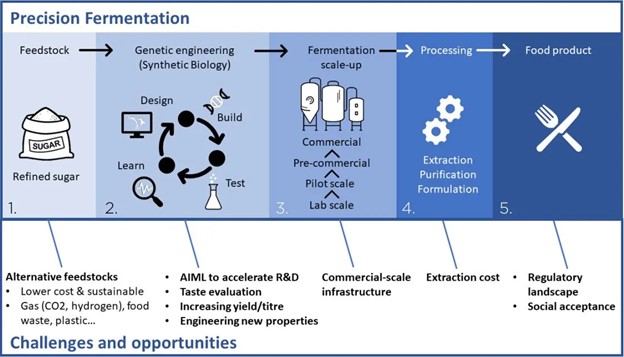
Fermentation has been used in food production for millennia. Ancient civilizations used microbial cultures to preserve foods, create alcoholic beverages, and improve the nutritional value and bioavailability of foods ranging from kimchi to tempeh. Over the past century, the role of fermentation has expanded far beyond its historical usage to a much broader range of applications. Fermentation now spans industrial chemistry, biomaterials, therapeutics and medicine, fuels, and advanced food ingredients. The suite of tools developed through fermentation’s evolution is now poised to revolutionize the food sector by accelerating the rise of alternative proteins. The term “fermentation” carries distinct meanings across different disciplines.
When you want to produce certain foods and beverages, a chemical process known as fermentation must occur. Fermentation is a type of biochemical reaction that’s able to extract energy from any carbohydrates in a solution without needing to use oxygen. Many organisms use the fermentation process to live. Along with foods and beverages, some additional products that can be created with the fermentation process include hydrogen gas, lactic acid, and ethanol. This metabolic process occurs when an organism converts any carbohydrate into an acid or alcohol. The carbohydrate can be sugar or starch. Yeast is able to perform fermentation by converting sugar directly into alcohol.
Exposing your fermenting food to air can not only prevent proper fermentation from taking place but also increase the risk of spoilage and food poisoning. There are a number of ways to prevent this. Aside from being a part of many recipes, submerging fermenting food in brine (a salt solution) prevents it from coming into contact with the air. This method works for solid pieces of food like chopped vegetables. The exact ph of the fermentation, which governs how much oxygen will be present , can also be controlled through the addition of vinegar. Storage many home fermenters use containers such as a mason jar with a lid or storage containers where food can be sealed and stored for long periods without air contamination.
Products of fermentation [ edit ]
Stanbury, p. F. , whitaker, a. , and hall, s. J. “an introduction to fermentation processes. ” principles of fermentation technology, 3rd edition, butterworth-heinemann, elsevier ltd. , 2017, pp. 1–20. Hutkins, r. W. “bread. ” microbiology and technology of fermented foods, 2nd edition, john wiley & sons ltd, 2019, pp. 301–342. Lee, b. H. “yeast-based processes and products. ” fundamentals of food biotechnology, 2nd edition, john wiley & sons ltd, 2015, pp. 207–237. Hui, y. H. , et al. “fermented cereal products. ” handbook of food and beverage fermentation technology, marcel dekker, inc. , 2004, pp. 712–799.